Metals | Free Full-Text | Resistivity and Passivity Characterization of Ni-Base Glassy Alloys in NaOH Media
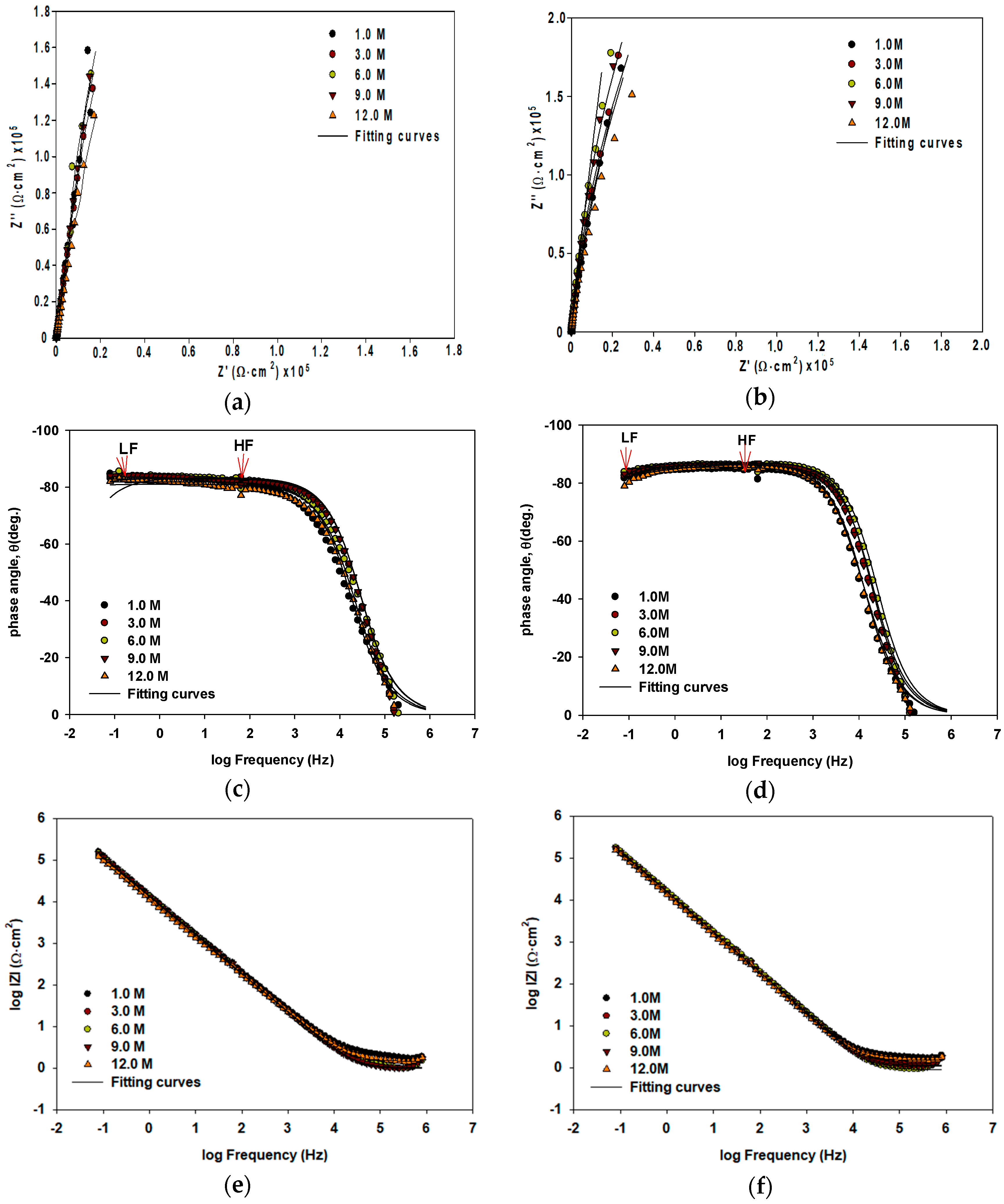
Metals | Free Full-Text | Resistivity and Passivity Characterization of Ni-Base Glassy Alloys in NaOH Media

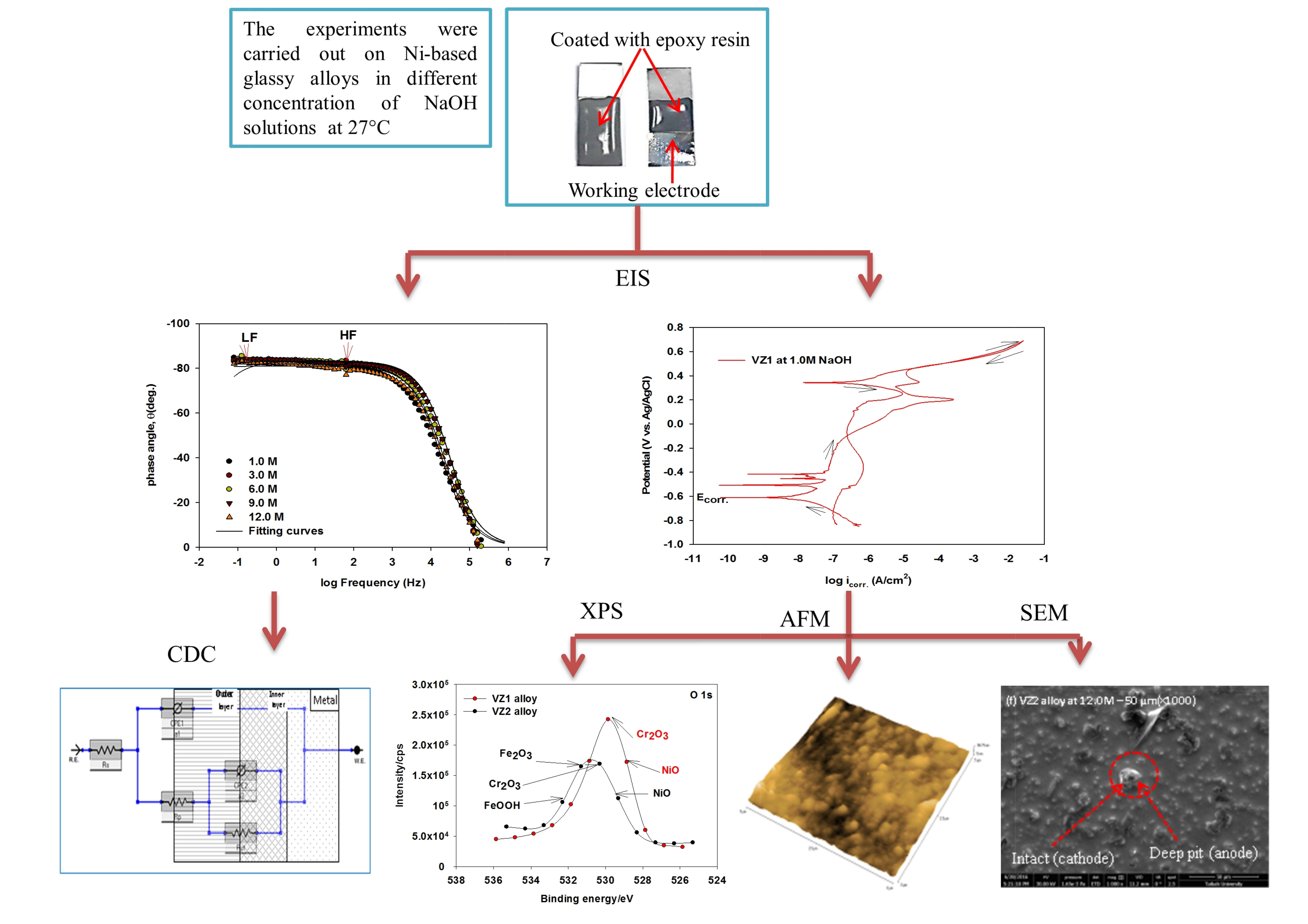
Metals | Free Full-Text | Resistivity and Passivity Characterization of Ni-Base Glassy Alloys in NaOH Media
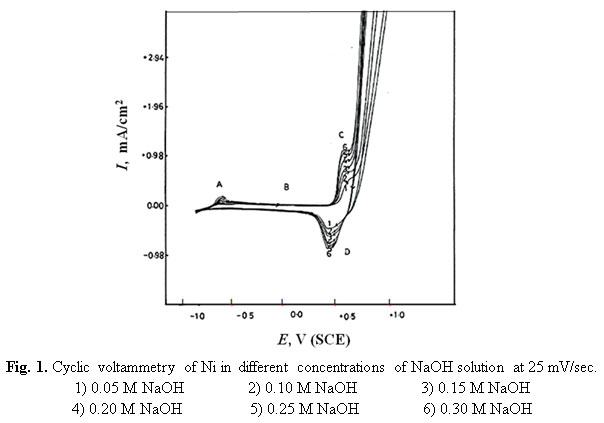
Corrosion Performance of Stainless Steel and Nickel Alloys in Aqueous Sodium Hydroxide as Revealed from Cyclic Voltammetry and Potentiodynamic Anodic Polarization : Oriental Journal of Chemistry

Engineering heterostructured Ni@Ni(OH)2 core-shell nanomaterials for synergistically enhanced water electrolysis - ScienceDirect

Find out the solubility of Ni(OH)2 in 0.1 M NaOH.Given that the ionic product of Ni(OH)2 is 2×10-15 - YouTube

Nickel hydroxide precipitate formed by adding sodium hydroxide (NaOH) to a solution containing nickel ions. Nickel hydroxide (Ni(OH)2) is precipitated Stock Photo - Alamy

Figure 5 from Thermodynamic model of Ni(II) solubility, hydrolysis and complex formation with ISA | Semantic Scholar