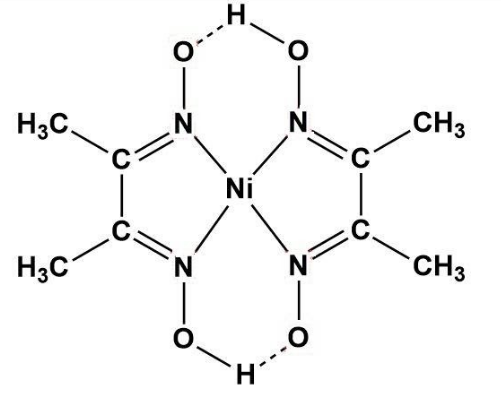
An alcoholic solution of dimethylglyoxime is added to an aqueous solution of nickel (II) chloride. Slow addition of ammonium hydroxide leads to the precipitation of a rosy red coloured metal complex. Then

Given below are two statements:Statement I: The identification of Ni2+ is carried out by dimethylglyoxime in the presence of NH4OHStatement II: The dimethylglyoxime is a bidentate neutral ligand.In the light of the
Ni^2+ can be estimated by used dmg and forms a Rosy red ppt. the complex is extra stabilised by which bonds ?

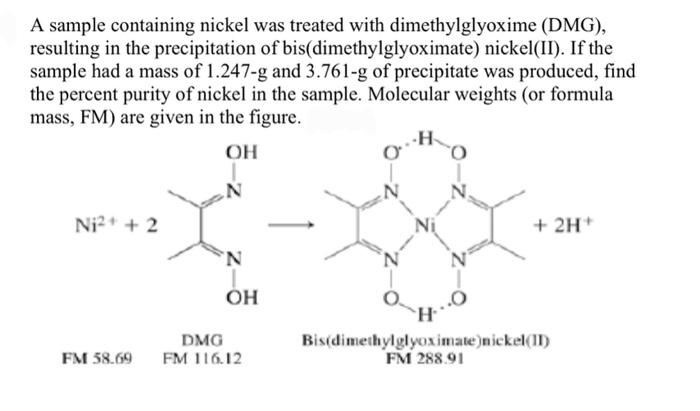
To estimate the amount of nickel as ni dmg in the solution of nickel chloride in presence of copper | PDF



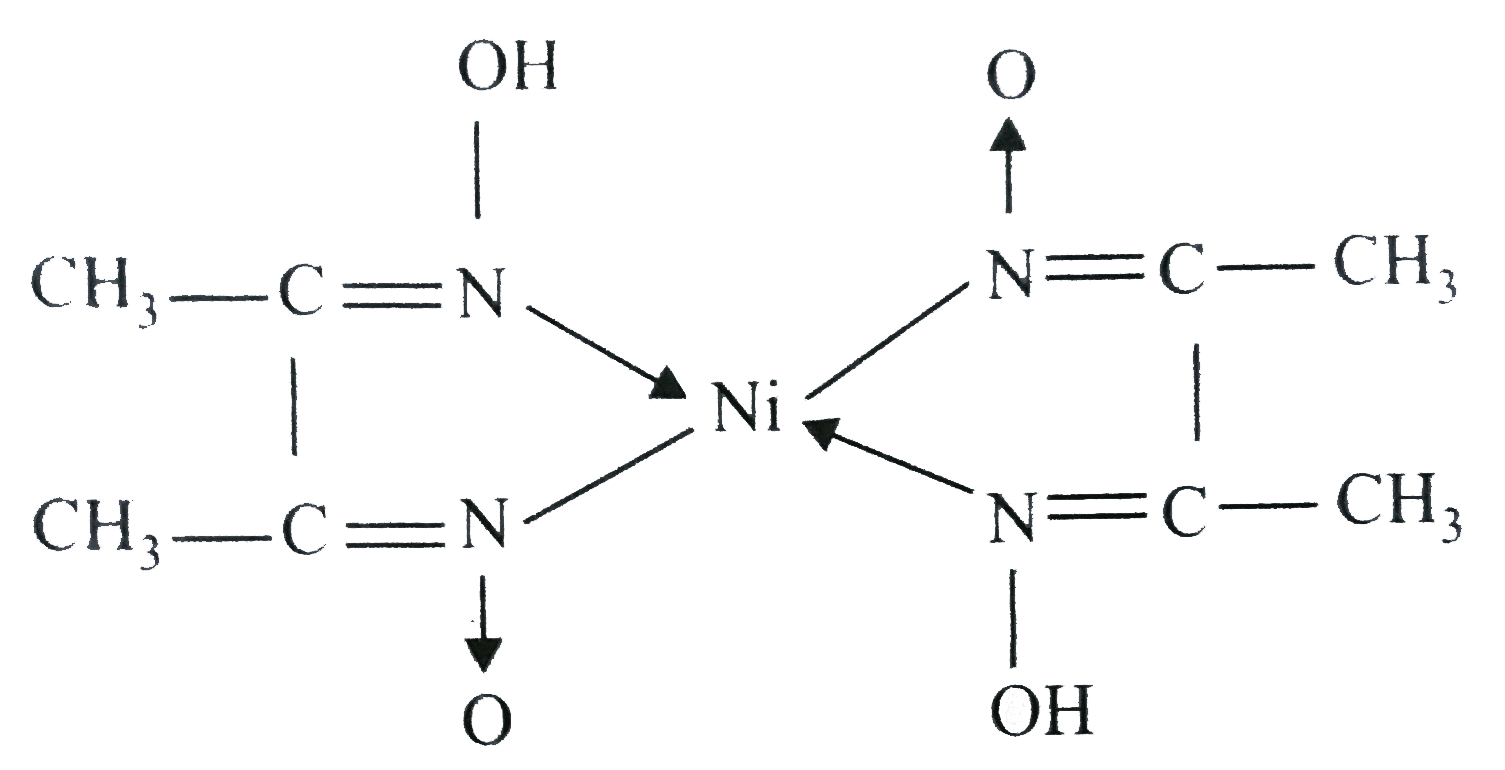
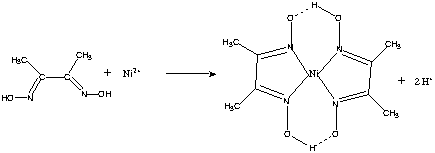







![The IUPAC name of the complex Ni[C(4)H(7)O(2)N(2)] formed by the react The IUPAC name of the complex Ni[C(4)H(7)O(2)N(2)] formed by the react](https://d10lpgp6xz60nq.cloudfront.net/physics_images/RS_P2_CHM_C09_E01_015_S01.png)
