![Both [Ni(CO)4] and [Ni(CN)4]2− are diamagnetic. The hybridization of nickel in these complexes, respectively, are : Both [Ni(CO)4] and [Ni(CN)4]2− are diamagnetic. The hybridization of nickel in these complexes, respectively, are :](https://search-static.byjusweb.com/question-images/toppr_ext/questions/347646_32360_ans_241c88579f7a422aa5a21574b50a4e4e.png)
Both [Ni(CO)4] and [Ni(CN)4]2− are diamagnetic. The hybridization of nickel in these complexes, respectively, are :

Nickel Carbonyl, Preparation, Structure and Properties | Organometallic Chemistry | Inorganic Chem - YouTube

Explain bonding and geometry of Ni(CO)4 by valence bond theory (if in plus zero state it wi have its normal - Chemistry - Coordination Compounds - 14313495 | Meritnation.com

![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-2.png)
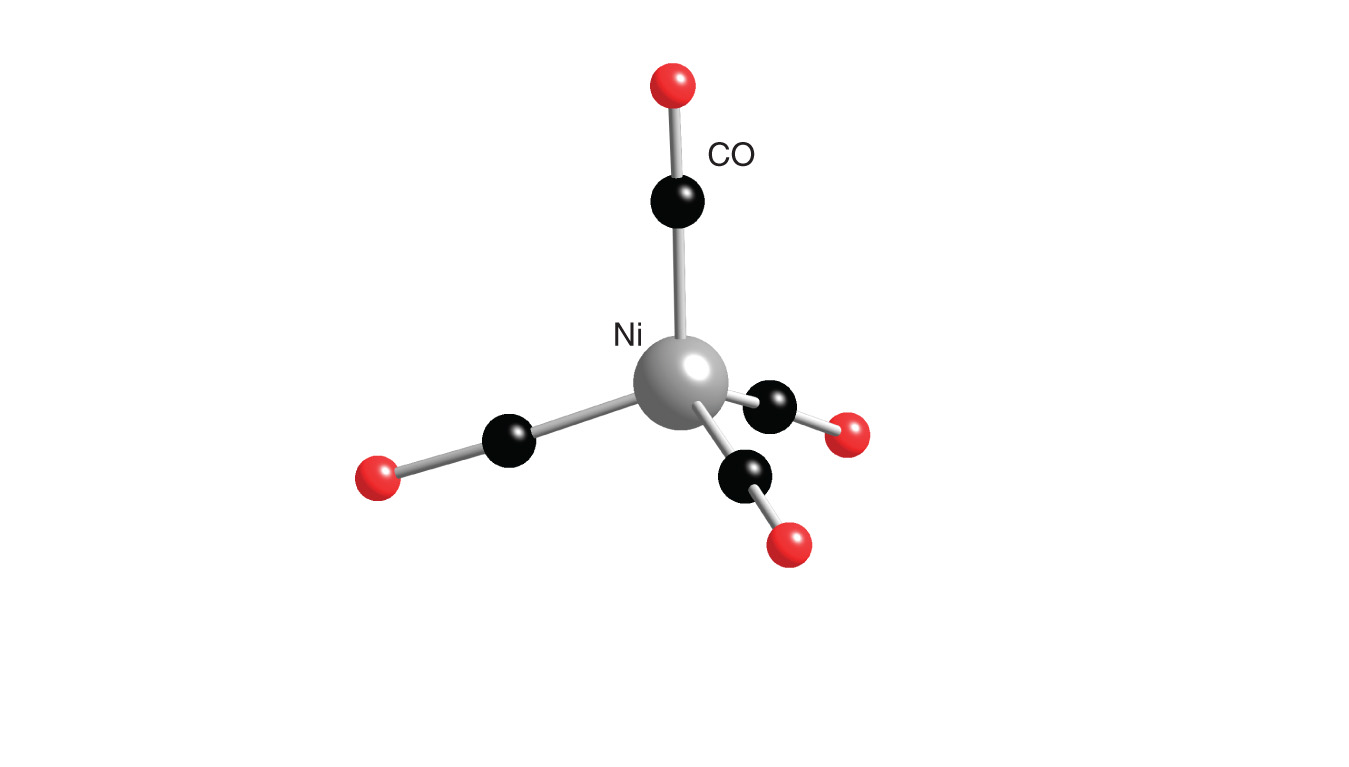
![Write the Hybridization and Magnetic Behaviour of the Complex [Ni(Co)4]. - Chemistry | Shaalaa.com Write the Hybridization and Magnetic Behaviour of the Complex [Ni(Co)4]. - Chemistry | Shaalaa.com](https://www.shaalaa.com/images/_4:0c935226f0c2451f9878137028e5360f.png)

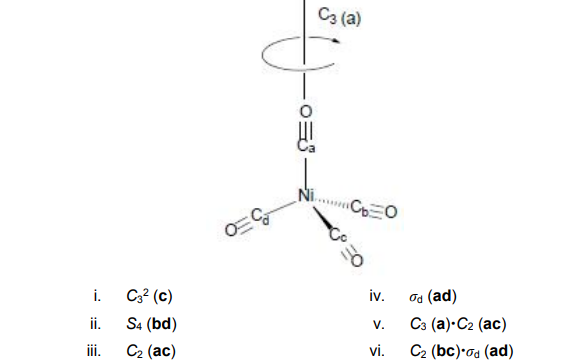
![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-1.png)
4.png)



![The geometry of [Ni(CO)]4 is? - askIITians The geometry of [Ni(CO)]4 is? - askIITians](https://files.askiitians.com/cdn1/cms-content/common/www.askiitians.comonlinetestforumsimages261-2458_3449166.png.jpg)



![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-3.png)
